
PPT Chemistry of Oscillating ColorChanging Reactions PowerPoint Presentation ID582379
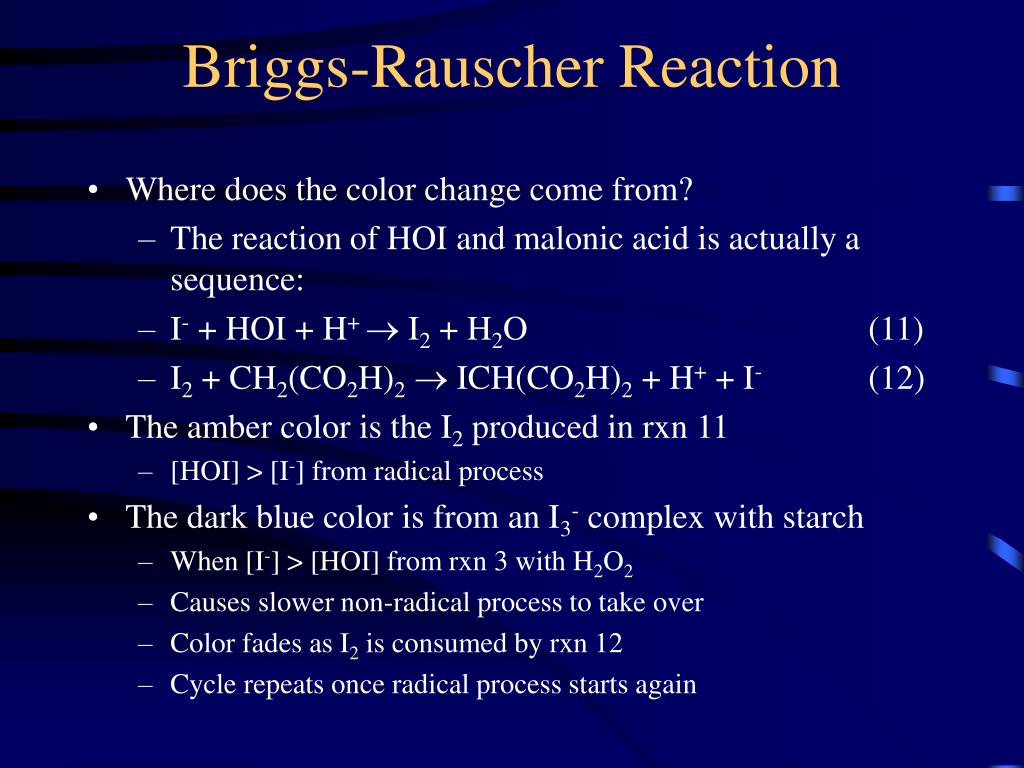
B ("radical process"): A fast process involving manganese and free radical intermediates, which converts hydrogen peroxide and iodate to free iodine and oxygen. This process also can consume iodide up to a limiting rate. IO 3- + 2 H 2 O 2 + CH 2 (CO 2 H) 2 + H + -> ICH (CO 2 H) 2 + 2 O 2 + 3 H 2 O. The striking visual demonstration occurs by.

BriggsRauscher Tepkimesi (The BriggsRauscher Reaction) YouTube
The reason for this is that in the first reaction, molecular iodine forms, which gives the solution an amber color. The iodine then turns into triiodide ions, which bond with starch and form a complex compound of a dark blue color. Parallel with this process, a reaction takes place between molecular.

Table 1 from Modeling of interactions between iodine interphase transport and oxygen production
The Briggs-Rauscher reaction, also known as 'the oscillating clock', is one of the most common demonstrations of a chemical oscillator reaction. The reaction begins when three colorless solutions are mixed together. The color of the resulting mixture will oscillate between clear, amber, and deep blue for about 3-5 minutes.
PPT Chemistry of Oscillating ColorChanging Reactions PowerPoint Presentation ID582379
There's something about an oscillating reaction that really makes students' jaws drop and gets them asking questions. Among the many types of such reactions,.

Briggs Rauscher Reaction ChemTalk
The Briggs-Rauscher reaction, also known as 'the oscillating clock', is one of the most common demonstrations of a chemical oscillator reaction. The reaction.

Pin on Chemistry
How To Do Briggs-Rauscher Reaction At Home DiyDon't Forget To Subscribe UsWebsite: http://diyfactoryworld.blogspot.com/Like Facebook: https://.

BriggsRauscher Reaction YouTube
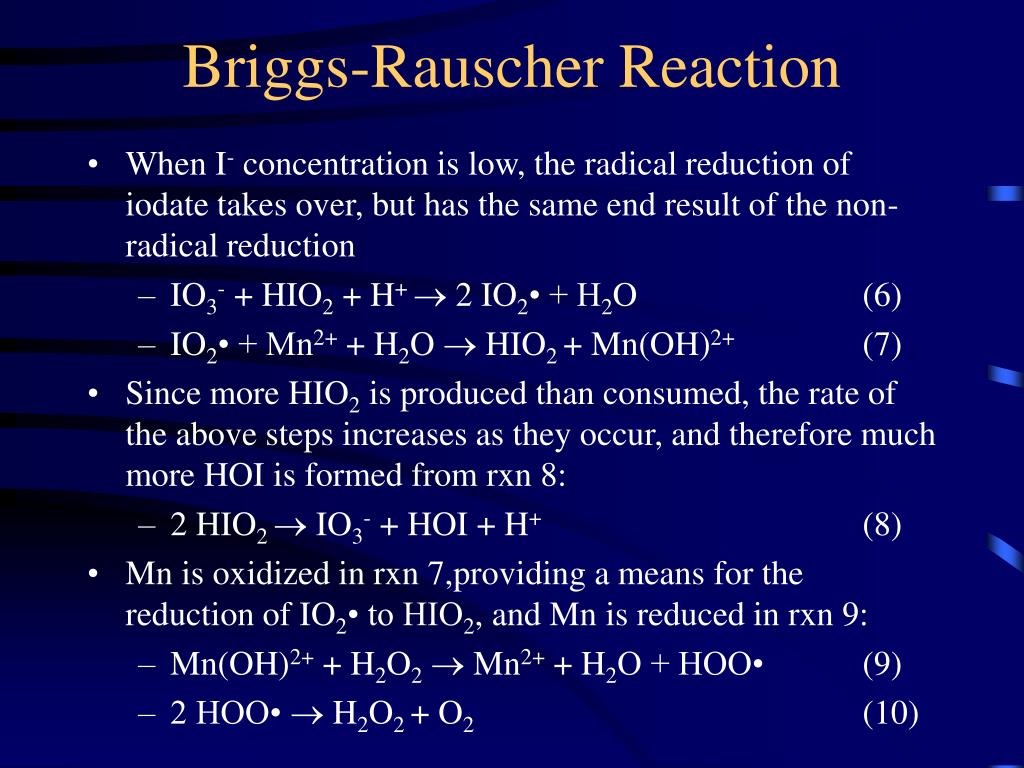
This sequence of color changes will repeat with a period of approximately 15 seconds at 25 oC. The reaction last about 5 min. The Briggs-Rauscher Reaction. IO3- + 2 H2O2 + CH2(CO2H)2 + H+ --> ICH(CO2H)2 + 2 O2 + 3 H2O (1) This reaction can be broken into two component reactions:

Black & White Magic (BriggsRauscher Reaction) YouTube
The Briggs-Rauscher oscillating reaction is one of a small number of known oscillating chemical reactions. It is especially well suited for demonstration purposes because of its visually striking color changes: the freshly prepared colorless solution slowly turns an amber color, suddenly changing to a very dark blue.

Simulated behaviour of [I À ] vs. time of the acetonebased BR... Download Scientific Diagram
ABSTRACT: The Briggs Rauscher reaction is a popular − demonstration to illustrate chemical oscillations in laboratories, classrooms, and public seminars because of its simplicity and visual appeal. Here, we adapt the Briggs Rauscher reaction to present − reaction diffusion convection patterns in the undergraduate − general or physical.

BriggsRauscher Reaktion Flaskman
The Briggs-Rauscher oscillating reaction is one of the very few oscillating reactions that we know of. Throughout the reaction, the concentration of iodide (.

X870 BriggsRauscher Reaction Oscillating Clock Lecture Demonstration Manual General
The reactions involved in the BriggsndashRauscher BR mechanism are the followingwhere is malonic acidSuppose these reactions take place in an open reactor Then under certain circumstances the color of the solution in the reactor shows regular oscillation between amber and dark blueThis Demonstration simulates the BR mechanism in a flow reactor.
PPT Chemistry of Oscillating ColorChanging Reactions PowerPoint Presentation ID582379
Briggs-Rauscher oscillating reaction is one of a small number of known oscillating chemical reactions. It is especially well suited for demonstration purposes because of its visually striking colour changes: the freshly prepared colourless solution slowly turns an amber colour, then suddenly changes to a very dark blue.

Briggs Rauscher Oscillating Chemical Reaction YouTube
MIT Chemistry Behind the MagicView the complete course: http://ocw.mit.edu/behindthemagicInstructor: John Dolhuh, Jessica HarropLicense: Creative Commons BY-.

Briggsrauscher Oscillating Reaction Photograph by Science Photo Library Pixels
While there are a variety of such reactions out there, perhaps the most iconic is the Briggs-Rauscher reaction. The rotating orange-black-colourless changes are incredibly reliable and the solutions, once made up, can tolerate a wide spectrum of concentrations.

Briggsrauscher Oscillating Reaction Photograph by Science Photo Library Pixels
.more Shop the NileRed store This is an interesting reaction with quite drastic color changes. It is also one of the few known oscillation reactions.I followed the prepartion guide from.
Solved 7. The BriggsRauscher reaction is an oscillating
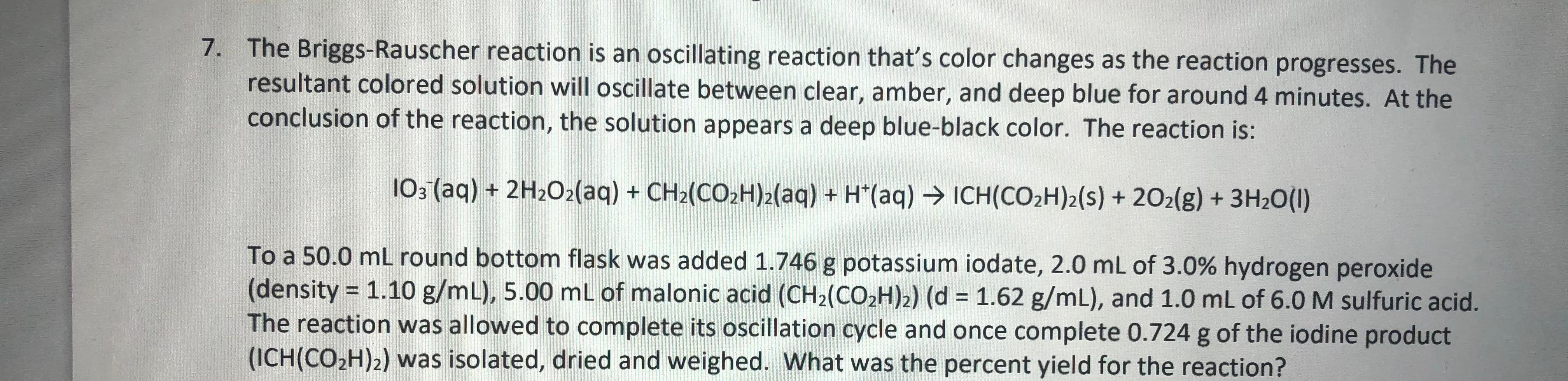
The oscillations of the Briggs-Rauscher reaction depend on the presence of free radicals in the solution. Adding antioxidants has been reported to change the oscillations due to their ability to "scavenge" the free radicals. Devise a way to use the Briggs-Rauscher reaction to test a chemical's ability to function as an antioxidant.